A) 1.03 V
B) 1.12 V
C) 1.24 V
D) 0.36 V
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the following reaction: Na++ e − → Na Which of the following best describes the reaction?
A) Oxidation half-reaction
B) Reduction half-reaction
C) Ionization
D) Dissociation
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the following reaction: Cu → Cu 2+ + 2e - Which of the following best describes the reaction?
A) Oxidation half-reaction
B) Reduction half-reaction
C) Hydrolysis
D) Excitation
F) A) and D)
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
What is the cell potential (E0) f or a galvanic cell formed from the following two half-reactions? Assume that the cell temperature is 38°C and the operating pressure is 0.04 atm. 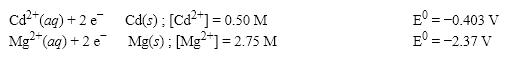
A) 1.90 V
B) 1.95 V
C) 1.99 V
D) 2.20 V
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In a galvanic cell, reduction occurs at _____.
A) the cathode
B) a salt bridge
C) the anode
D) the external circuit
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many grams of silver are deposited at a platinum cathode in the electrolysis of AgNO 3 (aq) by 5.30 A of electric current in 4.0 hours?
A) 85.3 g
B) 42.6 g
C) 121 g
D) 188 g
F) None of the above
Correct Answer

verified
Correct Answer
verified
True/False
The standard hydrogen electrode (SHE)involves a platinum wire or foil that is the conducting source of electrons; hydrogen gas is bubbled over the electrode at a pressure of 1 atm, and the electrolyte solution is 1 M HCl(aq).
B) False
Correct Answer

verified
Correct Answer
verified
True/False
All dry cell batteries are rechargeable.
B) False
Correct Answer

verified
Correct Answer
verified
True/False
The species undergoing reduction is referred to as an oxidizing agent.
B) False
Correct Answer

verified
Correct Answer
verified
True/False
If a chemical species gains electrons in a redox reaction, the species is said to have undergone oxidation.
B) False
Correct Answer

verified
Correct Answer
verified
True/False
In the context of the cell notation of galvanic cells, the anode is always written on the right and the cathode on the left.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A salt bridge between half-reactions maintains the electrical balance of a galvanic cell. This bridge is filled with:
A) a strong electrolyte.
B) inert carbon.
C) a weak electrolyte.
D) inert helium.
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the cell potential (E 0 ) for a galvanic cell formed from the following two half- reactions? 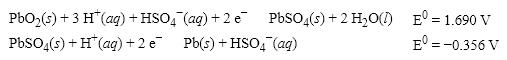
A) +1.334 V
B) − 2.046 V
C) +2.046 V
D) +2.758 V
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the balanced chemical equation for the following cell: 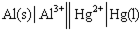
A) Al( s ) + Hg2+( aq ) → Al3+( aq ) + Hg( l )
B) 2 Al( s ) + Hg2+( aq ) → 2 Al3+( aq ) + Hg( l )
C) Al( s ) + 3 Hg2+( aq ) → Al3+( aq ) + 3 Hg( l )
D) 2 Al( s ) + 3 Hg2+( aq ) → 2 Al3+( aq ) + 3 Hg( l )
F) None of the above
Correct Answer

verified
Correct Answer
verified
True/False
In any galvanic cell, the half-reaction with the more positive reduction potential will be the cathode.
B) False
Correct Answer

verified
Correct Answer
verified
True/False
In the context of electrolysis, if the electrodes are chemically inert materials that simply provide a path for electrons, the process is called active electrolysis.
B) False
Correct Answer

verified
False
Correct Answer
verified
True/False
The Nernst equation describes the pH required for a spontaneous electrolytic reaction.
B) False
Correct Answer

verified
False
Correct Answer
verified
Multiple Choice
By convention, the standard hydrogen electrode (SHE) is assigned a voltage of _____ V .
A) − 1.000
B) 1.000
C) 0.150
D) 0.000
F) B) and C)
Correct Answer

verified
Correct Answer
verified
True/False
Reduction occurs at the anode of a galvanic cell.
B) False
Correct Answer

verified
Correct Answer
verified
True/False
The rusting of automobile bodies is an example of uniform corrosion.
B) False
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 45
Related Exams