A) I
B) II
C) III
E) A) and C)
Correct Answer

verified
Correct Answer
verified
Essay
Which of the following compounds is more acidic? Explain why. 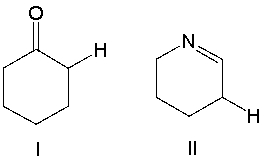
Correct Answer

verified
Compound I is more acidic. The conjugate...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Identify a Brønsted-Lowry acid.
A) proton acceptor
B) proton donor
C) species remaining after acid is deprotonated
D) species remaining after base is protonated
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Essay
For the following acid-base reaction, predict which side the equilibrium is favored. Explain why. 
Correct Answer

verified
Favors the right side.
Both the base and...View Answer
Show Answer
Correct Answer
verified
Both the base and...
View Answer
Multiple Choice
Which of the following compounds is most acidic? 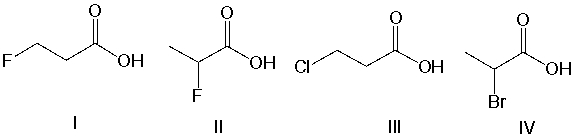
A) I
B) II
C) III
D) IV
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following compound is best classified as a ____. 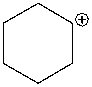
A) Brønsted-Lowry acid
B) Lewis acid
C) Brønsted-Lowry base
D) Lewis base
E) Both C & D
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Essay
For the following reaction label the acid, base, conjugate acid and conjugate base. 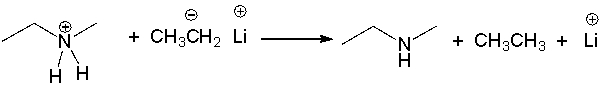
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds is most basic? 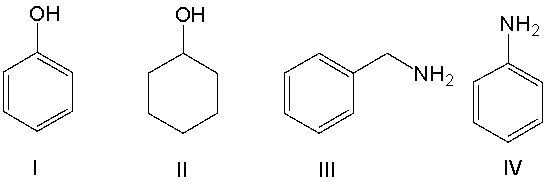
A) I
B) II
C) III
D) IV
E) All of these
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Essay
Determine if NaNH2 is a suitable reagent to deprotonate the following compound. Explain why. 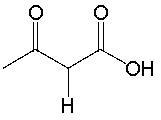
Correct Answer

verified
Yes.
The conjugate base is resonance sta...View Answer
Show Answer
Correct Answer
verified
The conjugate base is resonance sta...
View Answer
Multiple Choice
Which of the following compounds is most acidic?
A) HF
B) HCl
C) HBr
D) HI
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Rank the following compounds in decreasing order of acidity. 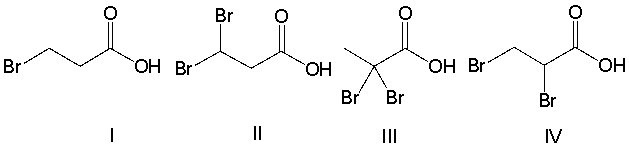
A) III > II > IV > I
B) II > IV > III > II
C) IV > III > II > I
D) IV > II > III > I
E) III > IV > II > I
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the following reaction, identify the Lewis acid. 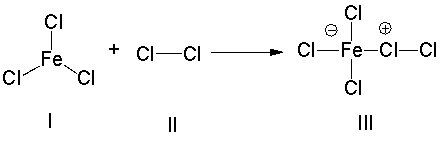
A) I
B) II
C) III
D) Both I & III
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the counterion in (CH3) 3CLi?
A) CH3+
B) CH3-
C) (CH3) 3C-
D) Li+
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Rank the following protons in decreasing order of acidity. 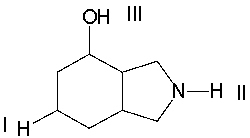
A) II > III > I
B) I > II > III
C) III > I > II
D) III > II > I
E) None of these
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Essay
Provide a curved arrow mechanism for the following acid-base reaction. 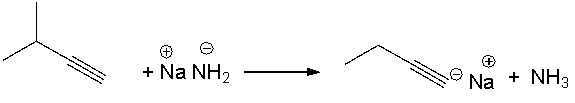
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the following acid-base reaction, predict which side of the equilibrium is favored. 
A) favor right side
B) favor left side
C) neither
E) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following solvents can be used with NaNH2?
A) CH3CH2OH
B) CH3OH
C) H2O
D) Liquid NH3
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is a cation?
A) a negatively charged ion
B) a positively charged ion
C) a sodium atom
D) a hydrogen molecule
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Essay
Draw the conjugate base of CH3C CH.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In a Brønsted-Lowry acid-base reaction, the reactants are _______.
A) Brønsted-Lowry acid and Brønsted-Lowry base
B) Brønsted-Lowry acid and conjugate base
C) Brønsted-Lowry base and conjugate acid
D) conjugate acid and conjugate base
E) Brønsted-Lowry acid and conjugate acid
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 127
Related Exams