A) 4
B) 9
C) 8
D) 7
E) 10
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 15-g sample of lithium is reacted with 15 g of fluorine to form lithium fluoride: 2Li + F2 2LiF.After the reaction is complete,what will be present?
A) 2.16 moles lithium fluoride only
B) 0.789 moles lithium fluoride only
C) 2.16 moles lithium fluoride and 0.395 moles fluorine
D) 0.789 moles lithium fluoride and 1.37 moles lithium
E) none of these
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If 45.0 g of O2 are mixed with 45.0 g of H2 and the mixture is ignited,what mass of water is produced?
A) 45.0 g
B) 50.7 g
C) 79.9 g
D) 25.3 g
E) 90.0 g
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What would be the g Al / mole S ratio for the product of a reaction between aluminum and sulfur?
A) 26.98 g Al / mol S
B) 53.96 g Al / mol S
C) 80.94 g Al / mol S
D) 17.99 g Al / mol S
E) 40.47 g Al / mol S
G) All of the above
Correct Answer

verified
D
Correct Answer
verified
Short Answer
Naturally occurring iron contains 5.82% 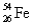 ,91.66%
,91.66% 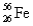 ,2.19%
,2.19% 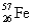 ,and 0.33%
,and 0.33% 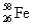 .The respective atomic masses are 53.940 amu,55.935 amu,56.935 amu,and 57.933 amu.Calculate the average atomic mass of iron.
.The respective atomic masses are 53.940 amu,55.935 amu,56.935 amu,and 57.933 amu.Calculate the average atomic mass of iron.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many of the following are true concerning balanced chemical equations? I.The number of molecules is conserved. II.The coefficients for the reactants tell you how much of each reactant you are given. III.Atoms are neither created nor destroyed. IV.The coefficients indicate the mass ratios of the substances used. V.The sum of the coefficients on the reactant side equals the sum of the coefficients on The product side.
A) 1
B) 2
C) 3
D) 4
E) 5
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the sum of the coefficients of the following equation when it is balanced using smallest whole numbers? NaNH2 + NaNO3 NaN3 +NaOH + NH3
A) 5
B) 6
C) 7
D) 8
E) 9
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A hypothetical element consists of two isotopes of masses 69.95 amu and 71.95 amu with abundances of 25.7% and 74.3%,respectively.What is the average atomic mass of this element?
A) 70.95 amu
B) 69.95 amu
C) 70.5 amu
D) 71.4 amu
E) 71.95 amu
G) All of the above
Correct Answer

verified
Correct Answer
verified
True/False
Oxides of copper include CuO and Cu2O.You heat 1.51 g of one of these copper oxides in the absence of air and obtain 1.21 g of Cu. True or false: You must have had CuO.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Naturally occurring element X exists in three isotopic forms: X-28 (27.977 amu,92.23% abundance) ,X-29 (28.976 amu,4.67% abundance) ,and X-30 (29.974 amu,3.10% abundance) .What is the identity of element X?
A) Cu
B) Al
C) Ni
D) Si
E) Sr
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many grams of H2O will be formed when 32.0 g H2 is mixed with 12.0 g of O2 and allowed to react to form water?
A) 13.5 g
B) 286 g
C) 6.8 g
D) 3.4 g
E) 144 g
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Balanced chemical equations imply which of the following?
A) Numbers of molecules are conserved in chemical change.
B) Numbers of atoms are conserved in chemical change.
C) Volume is conserved in chemical change.
D) A and B
E) B and C
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which compound contains the highest percent by mass of hydrogen?
A) HCl
B) H2O
C) H2SO4
D) H2S
E) HF
G) A) and C)
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
You take an aspirin tablet (a compound consisting solely of carbon,hydrogen,and oxygen) with a mass of 1.00 g,burn it in air,and collect 2.20 g of carbon dioxide and 0.400 g water.The molar mass of aspirin is between 170 and 190 g/mol.The molecular formula of aspirin is
A) C6H8O5
B) C9H8O4
C) C8H10O5
D) C10H6O4
E) none of these
G) A) and E)
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
How many grams of potassium are in 27.8 g of K2CrO7?
A) 4.49 g
B) 1.422 g
C) 8.98 g
D) 78.2 g
E) 55.6 g
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Give (in order) the correct coefficients to balance the following reaction: H2SnCl6 + H2S SnS2 + HCl
A) 1,2,1,6
B) 1,2,2,2
C) 1,1,1,6
D) 6,2,1,1
E) 2,4,2,6
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A substance,A2B,has the composition by mass of 60% A and 40% B.What is the composition of AB2 by mass?
A) 40% A,60% B
B) 50% A,50% B
C) 27% A,73% B
D) 33% A,67% B
E) none of these
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
SO2 reacts with H2S as follows: 2H2S + SO2 3S + 2H2O When 7.50 g of H2S reacts with 12.75 g of SO2,which statement applies?
A) 6.38 g of sulfur are formed.
B) 10.6 g of sulfur are formed.
C) 0.0216 moles of H2S remain.
D) 1.13 g of H2S remain.
E) SO2 is the limiting reagent.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the mass of 4 atom(s) of copper in grams?
A) 254.2 g
B) 2.37 1021 g
C) 9.57 10-24 g
D) 6.022 1023 g
E) 4.22 10-22 g
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A chemical reaction has the equation: 2A + B C.Which of the following figures best illustrates a stoichiometric ratio of A and B?
I. 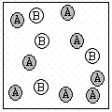 II.
II. 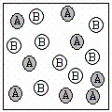 III.
III. 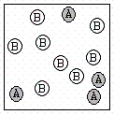 IV.
IV. 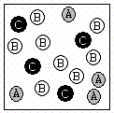
A) I only
B) II only
C) III only
D) IV only
E) both I and IV
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 134
Related Exams